Write the Isotopes of Hydrogen Explain Their Differences
This isotope of hydrogen contains 1 proton 1 electron and 1 neutron. It is also important to note that.
Hydrogen Isotopes Stock Illustrations 24 Hydrogen Isotopes Stock Illustrations Vectors Clipart Dreamstime
Tritium is a stable radioactive isotope or radioisotope of hydrogen and it has a half-life of about 123 years.
. 4 rows Name the isotopes of hydrogen. _ 1 3textrm H 1. Isotopes of Hydrogen and Carbon.
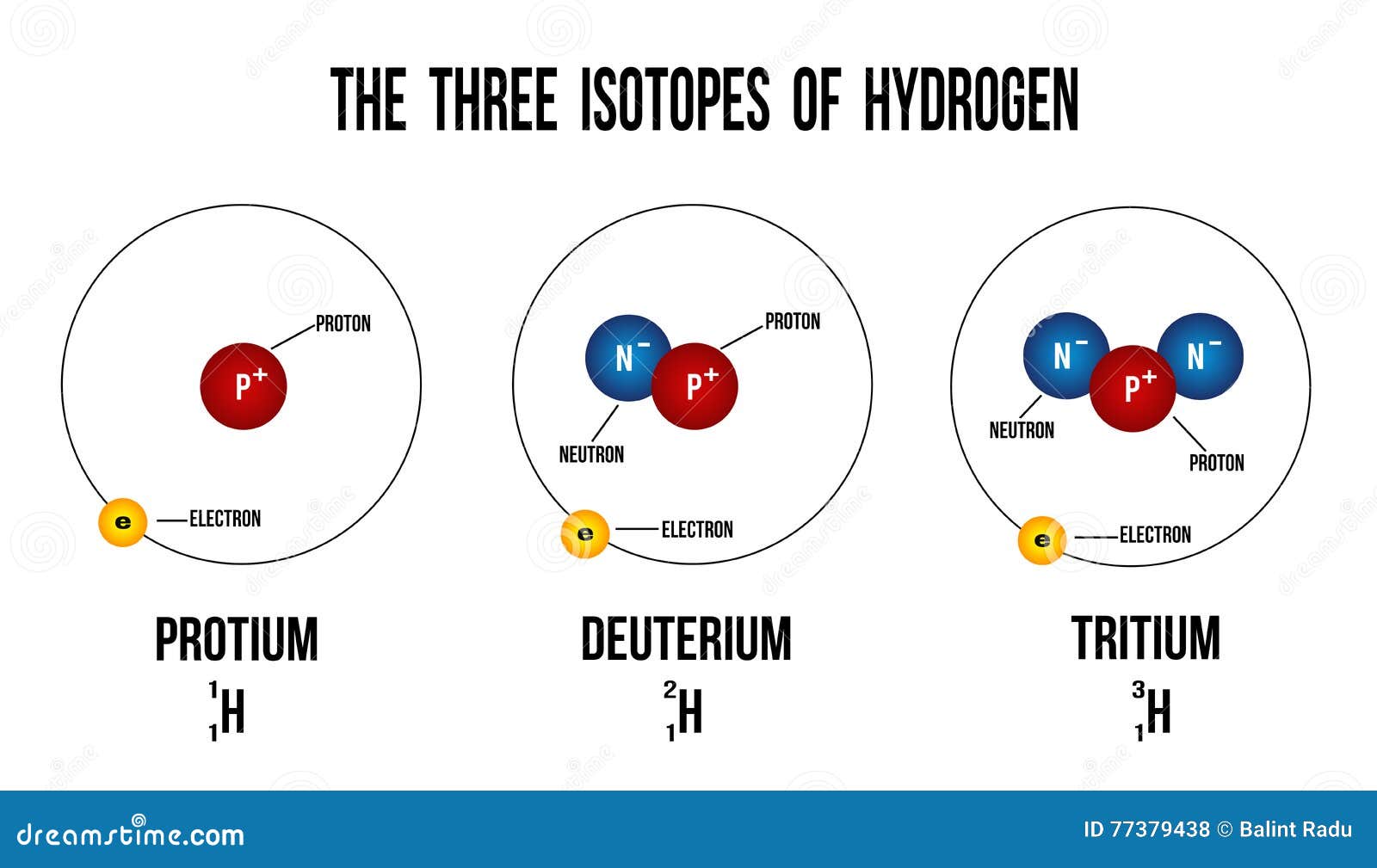
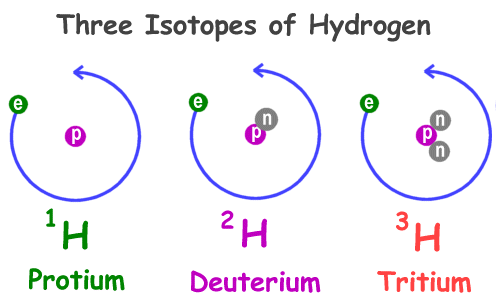
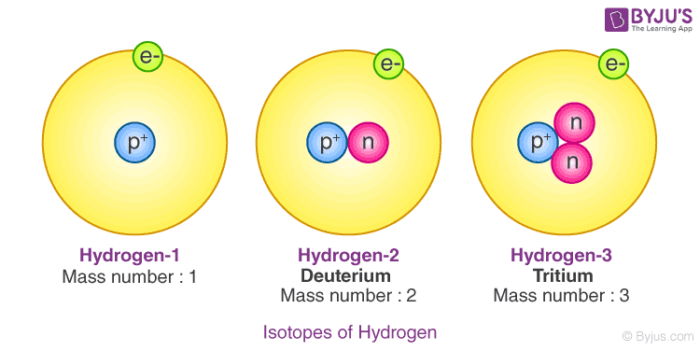
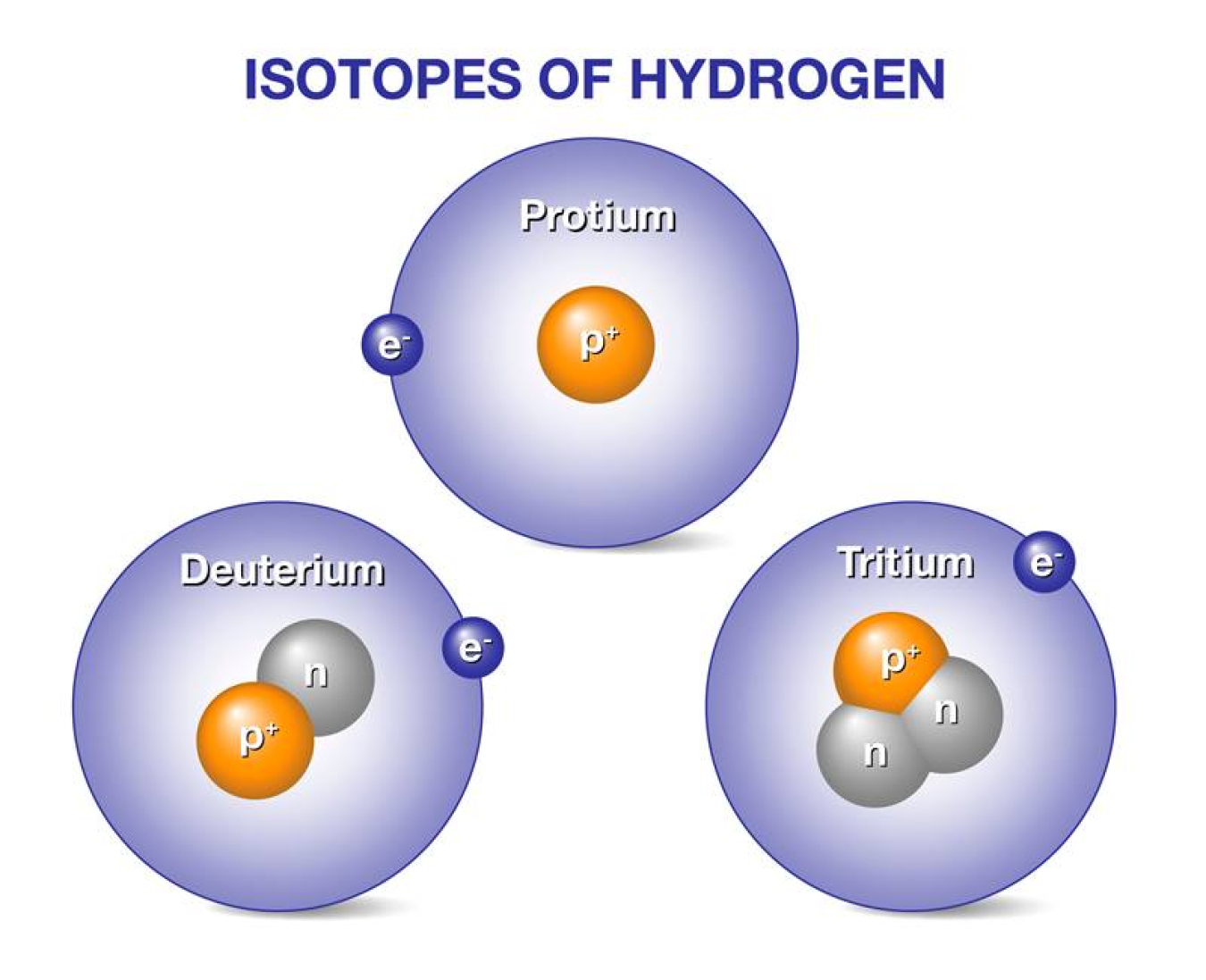
1 1 H 2 1 H 3 1 H. In addition to the reasons ste listed the isotopes of hydrogen have the greatest differences in mass compared to other elements. How many protons neutrons and electrons are present in each isotope.
This isotope of hydrogen contains 1 proton 1 electron and no neutrons. First used by Soddy. 234 92 U 235 92 U and 238 92 U.
Difference between Atom and molecule. The main difference between the three isotopes of Hydrogen are the number of neutrons in the nucleus. Isotopes have a different number of neutrons but they have the same number of protons and electrons.
All of the remaining radioactive isotopes have half-lives that are less than 27 s and the majority of these have half-lives that are less than 83 milliseconds ms. Isotope Examples Carbon 12 and Carbon 14 are both isotopes of carbon one with 6 neutrons and one with 8 neutrons both with 6 protons. The isotopes of an element are like different versions of an element - they have the same number of protons but.
Write a note on isotopes of Uranium. 3 - 1 2. The atomic number is the same as the number of protons and the atomic mass is the addition of neutrons and protons.
Isotopes of all elements can be used in kinetic isotope experiments. The atoms of these isotopes have one electron to balance the charge of the one proton. Hydrogen 1 H has three naturally occurring isotopes sometimes denoted 1 H 2 H and 3 H.
Write their atomic composition schematically and explain. Protium and Deuterium are classified under the stable isotopes of hydrogen. As we know that hydrogen element has three isotopes which are protium deuterium and tritium.
They have the same number of protons but have the different number of neutrons of each of the atom. 2 - 1 1. The number of neutrons in protium is zero.
Among the other heavier isotopes of hydrogen 5H is the most stable and 7H is the minimum stable. Their nuclear symbols are therefore 1 H 2 H and 3 H. Examples of radiogenic isotopes include argon-40 and hydrogen-4.
The atomic number is defined by the number of protons present in the atom. Protium deuterium and tritium are the three hydrogen isotopes. Explain their symbolic designation using the isotopes of hydrogen.
STRUCTURE OF THE ATOM 21 Activity 116 2. Read more. Hydrogen is the only element whose isotopes have.
Protium has zero neutrons while deuterium has one and tritium has two. It is defined as the element that have the same number of protons but have the different number of neutrons of each of the atom. The three isotopes of uranium are.
Difference Between Isotope and Isobar. Isotopes is a Greek word isos means same and tope means place. All three of them have a same number of protons but a different number of neutrons.
It can also be noted that this isotope of hydrogen is radioactive. Of these 5 H is the least stable while 7 H is the most. This isotope of hydrogen contains 1 proton 1 electron and 2 neutrons.
So the atomic mass of isotopes varies with the number of. The dramatic differences in mass among the hydrogen isotopes. They all have the same amount of protons however the number of neutrons varies.
For example the isotopes of hydrogen may be written. 1 H and 2 H are stable while 3 H has a half-life of 12322 years. This is because atoms of an element can differ in the number of neutrons Q.
In hydrogen isotopes the only tritium is radioactive which emits low-energy particles. Isotopes of an element have the same atomic number number of protons but different atomic mass numbers due to different number of neutrons in their nuclei. Hydrogen has three stable isotopes called protium deuterium and tritium.
Deuterium has one neutron and tritium has two. Hydrogen has no neutrons Deuterium has. However because of the significant variations in mass they have different physical.
Thirteen radioisotopes have been characterized with the most stable being 15 O with a half-life of 12224 s and 14 O with a half-life of 70606 s. These individual nuclides are called isotopes of that element. Consider that deuterium is twice as heavy as protium and tritium is three-times as heavy as protium.
It is defined as. Since chemistry depends on the interactions of protons with electrons the chemical properties of the isotopes are nearly. The heaviest one is tritium.
The isotopes of hydrogen have respectively mass numbers of one two and three. Heavier isotopes also exist all of which are synthetic and have a half-life of less than one zeptosecond 10 21 s. Hydrogen isotopes have a difference in their reaction rates but they all have similar chemical properties and the electronic configuration of isotopes is the same.
Isotopes are atoms of an element whose nuclei have the same atomic number but different mass number. Write the formulae of the compounds of hydrogen formed with sodium and chlorine. And in tritium it is two.
An isotope is named after the element and the mass number of its atoms. Hydrogen has no neutron deuterium has one and tritium has two neutronsThe isotopes of hydrogen have respectively mass numbers of one two and threeTheir nuclear symbols are therefore 1H 2H. In deuterium it is one.
Protium also known as ordinary hydrogen is the lightest isotope of hydrogen followed by deuterium also known as heavy hydrogen.
Isotopes Of Hydrogen Plutonium Deuterium Tritium With Examples Videos

Write The Names Of Isotopes Of Hydrogen What Is The Mass Ratio Of These Isotopes
Understanding The Outsized Effect Of Hydrogen Isotopes Department Of Energy

Isotopes Of Hydrogen Introduction To Chemistry

Answer The Following Question Name The Isotopes Of Hydrogen Write Their Atomic Composition Schematically And Explain Which Of These Is Radioactive Chemistry Shaalaa Com

Isotopes Of Hydrogen Teaching Chemistry Chemistry Math Help
What Is The Difference Between Allotropes And Isotopes Materials Science Engineering

The Three Isotopes Of Hydrogen Video Lesson Transcript Study Com

Atomic Structure The Periodic Table Isotopes And Average Atomic Mass Ppt Download

The Three Isotopes Of Hydrogen Video Lesson Transcript Study Com

The Three Isotopes Of Hydrogen Video Lesson Transcript Study Com
What Is The Radioactive Isotope Of Hydrogen Quora

The Three Isotopes Of Hydrogen Video Lesson Transcript Study Com

The Three Isotopes Of Hydrogen Video Lesson Transcript Study Com
Climate Science Investigations South Florida Temperature Over Time

Comments
Post a Comment